–News Direct–
Pharmaceutical manufacturing is the lynchpin to getting drug innovation to people. While compliance protocols are key to ensuring the smooth operation of the industry, they are beset by processes that are not fit for purpose and are hindering their ability to deliver life-saving drugs faster. Suffocated by paper-based records and legacy systems, the large-scale manufacturing of drugs is being hampered. Its little wonder that a staggering 2 out of 3 FDA warnings are for non-compliance related to procedures not being followed appropriately, unclear work instructions of methods, failure to review equipment usage logs, and lack of shared procedures between Quality Assurance and production departments. These matters are often missed and go unnoticed because of the conventional paper-based systems in the pharmaceutical industry.
Helping to make pharmaceutical manufacturing compliant, safer, and faster, tech startup Leucine is today announcing a $7M series A funding round led by Ecolab Inc., a strategic investor, to scale its Compliance Cloud platform globally. This round also saw participation from all existing investors, including Pravega Ventures, Axilor Ventures, Techstars, and angels.

Vivek Gera, founder, and CEO of Leucine, commented: Paper-based manufacturing records are the industry's achilles' heel, fueling not only regulatory nightmares but also ballooning production costs and inefficiencies. The legacy solutions are no better, with their extremely long implementation cycles and rigid, siloed applications that leave manufacturers in a lurch.
Leucine's Compliance Cloud serves as a digital twin of the pharma manufacturing shop floor, bringing real-time performance monitoring, compliance management, and actionable insights to the table. For pharma manufacturers, leveraging data can mean the difference between a successful batch and a costly recall. What differentiates Leucine from some of the legacy digitization tools is its AI-driven capabilities designed not only to digitize pharma manufacturing workflows faster but also to provide proactive insights that enable pharma companies to stay compliant and produce faster and in a cost-effective manner.
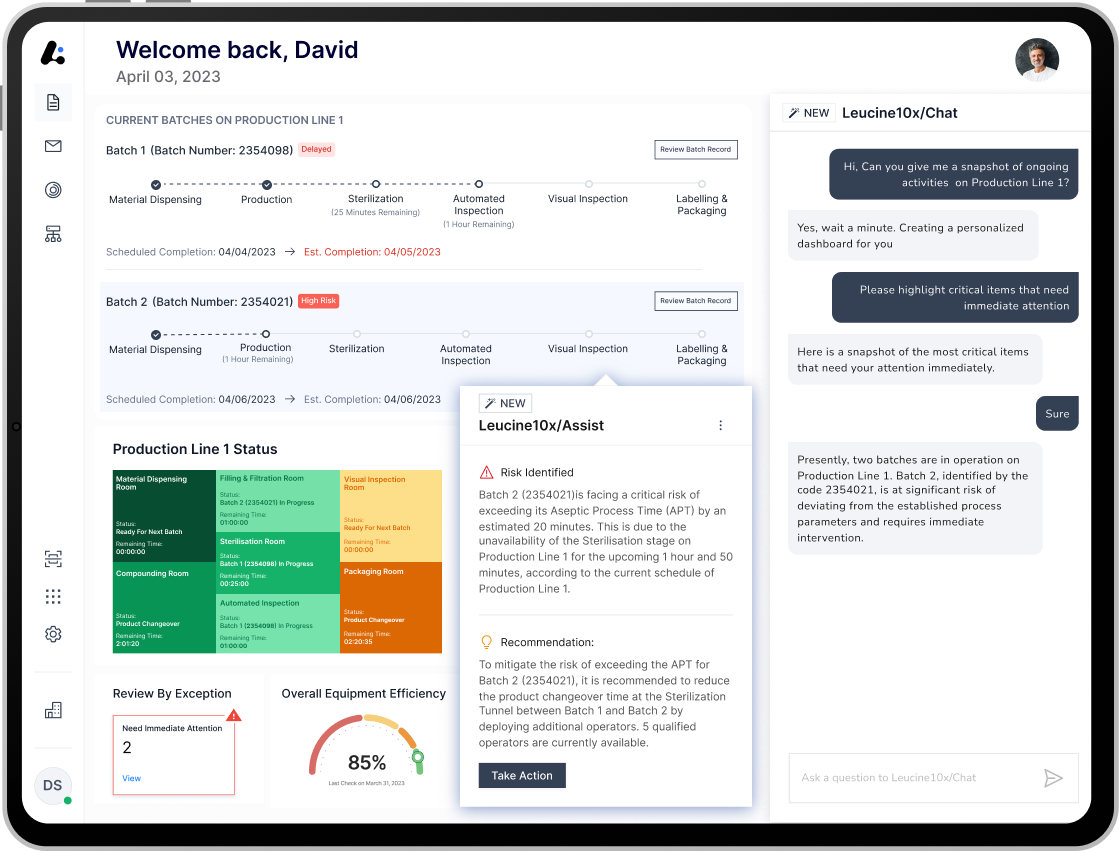
Leucine's platform can be implemented in 8 weeks. This speed of implementation becomes possible because of the proprietary AI-enabled digital process builder based on Large Language Models (LLMs) that rapidly digitizes paper SOPs into execution-ready digital formats. "Our models are trained on a wealth of pharma data, which allows the platform to create custom workflows enriched with GxP compliance measures, enabling us to deliver unparalleled value at breakneck speed," added Vivek Gera. A batch execution procedure is the single most important document in pharma manufacturing. This rapid digitization capability reduces the digitization cycle of a batch record from 6-8 months to 3-5 days.
Leucine is currently deployed at over 30 companies in over 300 pharma manufacturing facilities across 10 countries, including the US, India, Brazil, Mexico, UAE, and others.
The company is today also announcing the beta launch of Leucine10x, a groundbreaking AI framework designed to serve as a co-pilot in pharmaceutical manufacturing processes. Leucine10x will revolutionize how decision-making is done in pharma manufacturing, enabling Production and Quality Assurance teams to achieve their organization goals with ease and confidence. Built on proprietary LLM technology, Leucine10x offers an army of AI co-pilots that perform highly specialized tasks such as digitizing paper-based SOPs, creating a digital twin of the shop floor, collaborating with Production Managers in creating dynamic production plans, thus ensuring on-time batch delivery. Most significant is their ability to speed up Root Cause Analysis (RCA) of deviations in the manufacturing process by quickly analyzing data, including text-based records, logs, and even staff interview transcripts, to identify potential issues or patterns that might not be readily apparent.
Mustaq Singh Bijral, Co-founder and CPO of Leucine, said: "We're excited to share that Leucine10x is already operational in select customer facilities under a trusted tester agreement. The response has been incredibly positive, and due to high demand, we're currently enrolling new customers through a waitlist".
The funding round will support Leucine in refining its AI capabilities and making its AI Co-pilot a trusted partner to the production and quality managers on the shop floor and in expanding its reach to more facilities and customers.
Vivek and Mustaq have been relentless in their mission to make pharma manufacturing safer. This latest funding round will enable Leucine to bring the power of LLMs and associated technologies to make significant advances in pharma manufacturing. We couldnt be happier to support them in this quest said Rohit Jain, Co-founder and Partner at Pravega Ventures. Pravega Ventures also invested in the Seed round of Leucine.
Vivek Gera remarked: "We're just scratching the surface; our vision is to leverage AI and technology to bring safe medicine to patients across the world
About Leucine
Leucine is at the forefront of digitizing pharmaceutical manufacturing, leveraging cutting-edge AI technologies to bring unprecedented efficiency and compliance to the shop floor. Our innovative solutions, from Batch Planning to Batch Release, aim to modernize traditional procedures, replacing paper-based records with a seamless, digital-first approach. Trusted by industry leaders, Leucine not only streamlines the manufacturing process but also delivers actionable insights through its AI-driven analytics dashboard, empowering pharma companies to produce safer and more effective medicines. Leucine is headquartered in New Jersey, United States. For more information, visit https://www.leucine.io/
About Pravega Ventures
Pravega Ventures is an early stage Venture Capital fund that partners with passionate founders, who are leveraging technology to either disrupt existing markets or create new ones.
Contact Details
Leucine
Sampada Bhootna
+91 81458 07848
sampada.bhootna@leucinetech.com
Company Website
View source version on newsdirect.com: https://newsdirect.com/news/ai-startup-leucine-nets-7m-to-level-up-pharma-manufacturing-compliance-to-keep-pace-with-drug-innovation-929055468
Leucine
COMTEX_441968326/2655/2023-10-16T08:59:51
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Micro Trustiva journalist was involved in the writing and production of this article.

